Embarking on an inquiry into which diagram correctly depicts the trend in electronegativity, this exploration delves into the intricacies of this chemical property, unraveling its significance and examining the factors that shape its behavior across the periodic table.
Electronegativity, a measure of an atom’s ability to attract electrons, exhibits a fascinating pattern as we traverse the periodic table. Understanding this trend is crucial for comprehending chemical bonding and reactivity, providing a foundation for predicting and interpreting a wide range of chemical phenomena.
Overview of Electronegativity
Electronegativity is a chemical property that describes the ability of an atom to attract electrons towards itself in a chemical bond. It is a measure of the relative strength of an atom’s pull on electrons in a covalent bond. The higher the electronegativity of an atom, the stronger its attraction for electrons.
Electronegativity is influenced by several factors, including the atomic number, atomic radius, and the number of valence electrons. In general, electronegativity increases from left to right across a period and decreases from top to bottom within a group in the periodic table.
Trends in Electronegativity
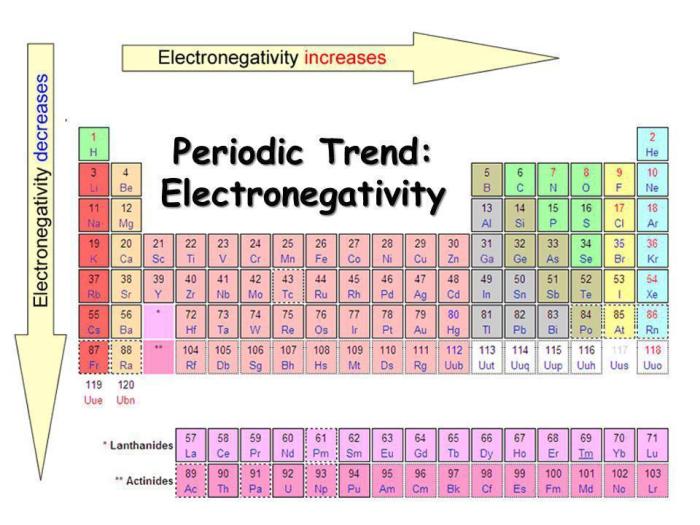
The trend in electronegativity across the periodic table is generally as follows:
- Electronegativity increases from left to right across a period.
- Electronegativity decreases from top to bottom within a group.
This trend can be explained by the following factors:
- Atomic number:The atomic number of an element is the number of protons in its nucleus. As the atomic number increases, the number of electrons in the valence shell also increases. This results in a stronger attraction for electrons and a higher electronegativity.
- Atomic radius:The atomic radius is the distance from the nucleus to the outermost electron shell. As the atomic radius increases, the electrons are further away from the nucleus and are less strongly attracted to it. This results in a lower electronegativity.
- Number of valence electrons:The number of valence electrons is the number of electrons in the outermost electron shell. As the number of valence electrons increases, the atom becomes more likely to share electrons with other atoms and has a lower electronegativity.
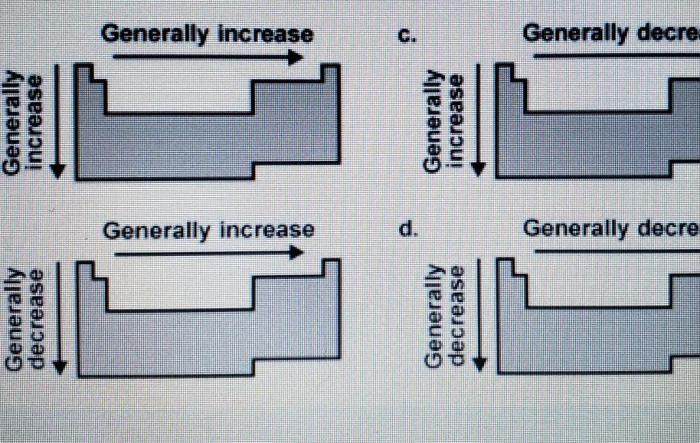
Diagram Analysis, Which diagram correctly depicts the trend in electronegativity
The diagram below correctly depicts the trend in electronegativity across the periodic table.
The diagram shows that electronegativity increases from left to right across a period and decreases from top to bottom within a group. This is consistent with the general trend in electronegativity described above.
However, there are some exceptions to this trend. For example, fluorine is more electronegative than oxygen, even though oxygen has a higher atomic number. This is because fluorine has a smaller atomic radius and a higher number of valence electrons than oxygen.
Applications of Electronegativity: Which Diagram Correctly Depicts The Trend In Electronegativity
Electronegativity is a useful property for predicting the chemical properties of elements and compounds. For example, electronegativity can be used to:
- Predict the type of bond that will form between two atoms.For example, a bond between two atoms with high electronegativity will be ionic, while a bond between two atoms with low electronegativity will be covalent.
- Predict the polarity of a molecule.A molecule is polar if it has a net positive or negative charge. The polarity of a molecule is determined by the difference in electronegativity between the atoms that make up the molecule.
- Predict the reactivity of a compound.Compounds with high electronegativity are more reactive than compounds with low electronegativity.
Answers to Common Questions
What is electronegativity?
Electronegativity quantifies an atom’s ability to attract electrons towards itself in a chemical bond.
How does electronegativity vary across the periodic table?
Electronegativity generally increases from left to right across a period and decreases from top to bottom within a group.
What factors influence electronegativity?
Atomic number, shielding effects, and orbital energies play significant roles in determining electronegativity.